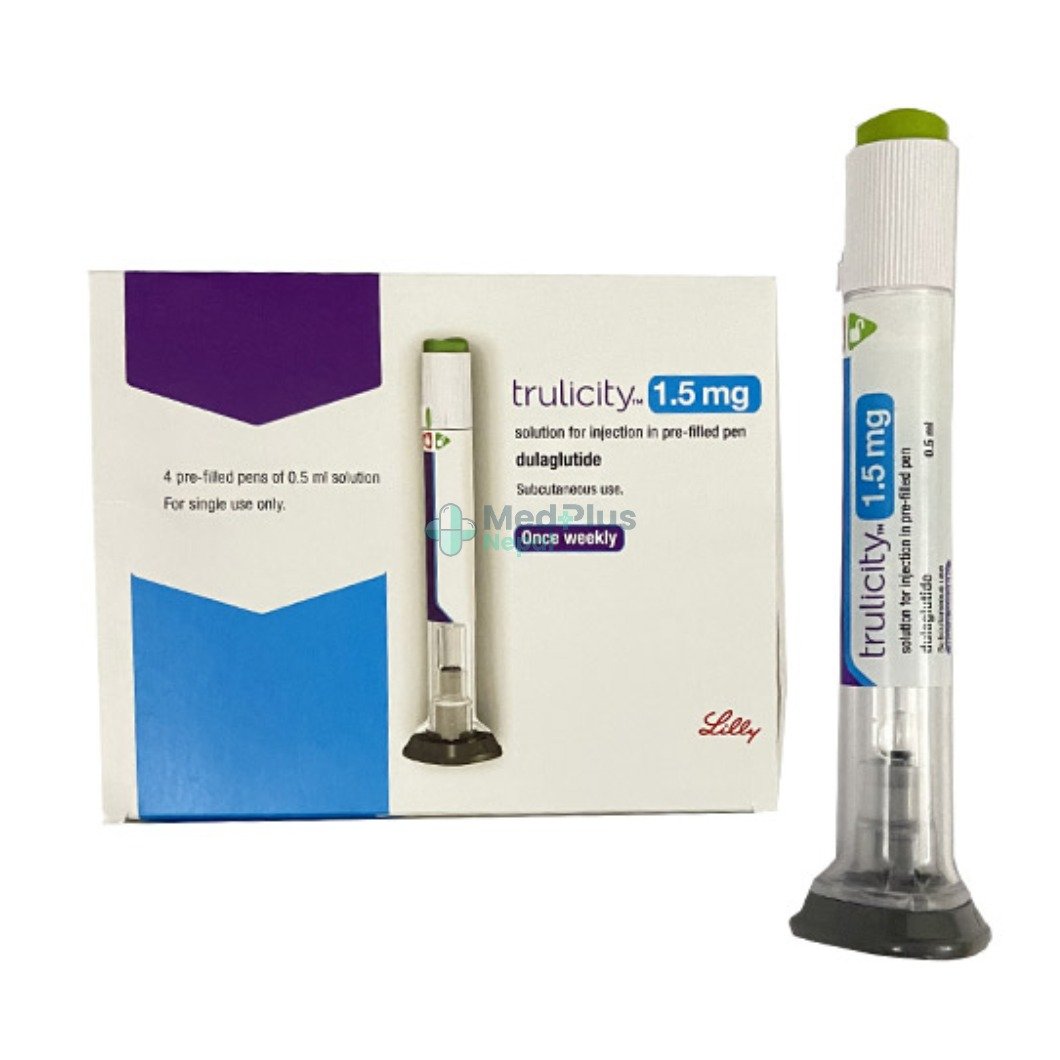
Trulicity 1.5mg ( 2 pre-filled pens per pack)
₨ 8,796.00
- Brand Name: Trulicity
- Manufacturer: Eli Lilly and Company
- Generic Name: Dulaglutide
- Class of Drug: Glucagon-Like Peptide-1 (GLP-1) Receptor Agonist
- Dosage Form: Solution for injection in pre-filled pens
- Strength: 1.5 mg per 0.5 mL
- Pack Size: 2 pre-filled pens per pack
- Uses: Treatment of type 2 diabetes mellitus to improve glycemic control
-
Mechanism of Action (MOA): Trulicity (dulaglutide) is a GLP-1 receptor agonist that enhances insulin secretion in response to meals, inhibits glucagon release, slows gastric emptying, and promotes satiety, thereby improving glycemic control in patients with type 2 diabetes mellitus.
-
Dosage and Administration:
- Recommended Dose: Initiate treatment with 0.75 mg once weekly. If additional glycemic control is needed, the dose may be increased to 1.5 mg once weekly. The maximum recommended dose is 1.5 mg once weekly.
- Administration: Inject subcutaneously in the abdomen, thigh, or upper arm. Trulicity can be administered at any time of day, with or without food.
- Missed Dose: If a dose is missed, administer as soon as possible if there are at least 3 days (72 hours) until the next scheduled dose. If less than 3 days remain, skip the missed dose and resume the regular weekly schedule. Do not administer two doses within 3 days.
-
Side Effects:
- Common: Gastrointestinal events such as nausea, vomiting, diarrhea, abdominal pain, and decreased appetite.
- Serious: Pancreatitis, thyroid C-cell tumors, acute kidney injury, and hypersensitivity reactions.
- Note: Side effects often diminish over time. Consult a healthcare provider if side effects persist or are severe.
-
Drug Interactions:
- Caution is advised when co-administering with other glucose-lowering agents to minimize the risk of hypoglycemia.
- Monitor renal function when used concomitantly with medications affecting renal function.
-
Precautions:
- Contraindications: Do not use in patients with a personal or family history of medullary thyroid carcinoma (MTC) or multiple endocrine neoplasia syndrome type 2 (MEN 2).
- Renal Impairment: Use with caution in patients with renal impairment.
- Pancreatitis: Discontinue if pancreatitis is suspected.
- Pregnancy and Breastfeeding: Consult a healthcare provider before use during pregnancy or breastfeeding.
- Alcohol: Limit alcohol consumption, as it may affect blood glucose levels







Reviews
There are no reviews yet.